It was a busy quarter for Arovella Therapeutics Ltd (ASX:ALA) in its aim to develop an invariant Natural Killer T (iNKT) cell therapy platform for cancer treatment.
The June quarter has set the company up to deliver several key milestones in the next 12 months including:
- Manufacture of clinical batches of lead product ALA-101 for phase 1 clinical trials;
- Securing a regulatory acceptance with the FDA/TGA to conduct a phase 1 clinical trial in CD19-positive blood cancers;
- Commencement of a phase 1 clinical trial for ALA-101 in patients with CD19-positive blood cancers;
- Presenting preclinical proof-of-concept data for its gastric cancer CLDN18.2-iNKT program; and
- Presenting preclinical proof-of-concept data for IL-12-TM as an armouring technology for Arovella’s CAR-iNKT cell platform.
Arovella is in a strong financial position to achieve these aims, with funding of $12.7 million as of June 30, 2024. This is expected to provide the company with enough funding to obtain preliminary data in its planned first-in-human clinical trial for ALA-101.
Critical milestone achieved
During the quarter, Arovella completed process development to define a clinic-ready manufacturing process for ALA-101 and the CAR-iNKT cell platform.
Arovella successfully developed a modular, semi-automated manufacturing process at Cell Therapies Pty Ltd for ALA-101. This patent-protected process is crucial for large-scale Good Manufacturing Practice (GMP) production, which is essential for regulatory approval and the commencement of phase 1 clinical trials.
The process ensures a high yield of Chimeric Antigen Receptor (CAR)-positive invariant Natural Killer T (iNKT) cells with exceptional purity. This advancement marks a significant step in advancing ALA-101 into clinical trials and establishing the CAR-iNKT cell platform for targeting various tumours.
The process significantly reduces technology transfer risks to new jurisdictions, utilising well-known automated cell therapy equipment, ensuring a well-controlled and reproducible GMP manufacturing process.
Arovella is now poised to proceed with engineering and GMP batches to produce the necessary material for its first-in-human clinical trials.
The following are the manufacturing process outcomes:
- High yield, >5,000-fold expansion of CAR-iNKT cells
- >60% of the cells have the CAR (i.e. CAR-iNKT cells);
- >99% purity of iNKT cells;
- Semi-automated, suitable for large-scale production;
- Lentiviral vector agnostic, meaning that a similar process can be used to generate additional products to target different cancer types;
- Potential to leverage FDA Platform Designation.
Importantly, the final product characteristics align with the expectations of global regulators, including the United States Food and Drug Administration (FDA), regarding product quality and safety.
The process preserves the beneficial highly cytotoxic CD4-negative population of invariant natural killer T (iNKT) cells, as described in Arovella's licensed patents.
These cells have demonstrated greater cytotoxicity than CD4-positive cells, as presented at the American Association for Cancer Research (AACR) Annual Meeting. It is anticipated that a balanced product with a mix of these cell phenotypes may enhance efficacy.
Achieving this milestone will facilitate the expansion of Arovella’s pipeline for its chimeric antigen receptor (CAR)-iNKT cell platform.
The manufacturing process can be applied to Arovella’s future CAR-iNKT cell products, significantly reducing the time required to progress from proof-of-concept data to clinical manufacture for programs with new CARs.
As the manufacturing process is lentiviral vector agnostic, it allows the incorporation of newly developed CARs or CARs from other programs capable of recognizing different tumour types, which can be added to iNKT cells, such as Claudin 18.2-targeting CAR-iNKT cells.
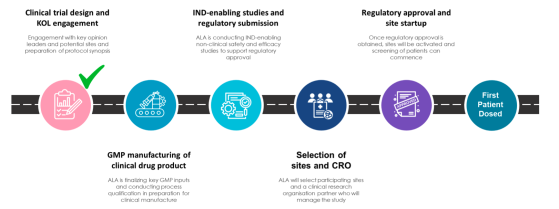
Preparing for human trials
Arovella has started preparing for its phase 1 first-in-human clinical trial to treat patients with CD19-positive blood cancers. During FY25, Arovella will focus on bringing ALA-101 into the clinic. The key activities to be conducted over the coming months can be seen below:
Further achievements this quarter
In April, Arovella presented new pre-clinical data on ALA-101 at the AACR Annual Meeting in San Diego. The data detailed two distinct phenotypes of cells within the drug product, each responding differently to tumour cells.
ALA-101 CAR-iNKT cells were classified based on CD4 presence (CD4+ vs CD4-). CD4- cells exhibited higher tumour cell-killing ability via the CD19 Chimeric Antigen Receptor (CAR), while CD4+ cells showed faster proliferation in response to CD19+ tumour cells.
Different cytokine responses were also noted between the two cell types following CAR activation. These findings suggest that having diverse CAR19-iNKT cell subsets could be beneficial in treating CD19+ cancers.
Arovella's iNKT manufacturing method aims to maintain a balance of these cell populations, particularly the cytotoxic CD4-cells. More information is available on the company's website.
Arovella also strengthened its team and Scientific Advisory Board with notable appointments. Dr Michelle Ferguson, with a PhD in Immunology and 15+ years of experience in academia and industry, joins as director of Research and Development while Dr Kelvin Yip, an expert in tumour biology with a PhD from the University of Melbourne, joins as associate director of Research and Development.
Additionally, Professor Gianpietro Dotti, a pioneer in CAR-iNKT cell strategies for cancer treatment, has been appointed to the Scientific Advisory Board. Professor Dotti's extensive research in engineered immune cells for cancer treatment includes more than 200 peer-reviewed articles and multiple Highly Cited Researchers awards.
Read more on Proactive Investors AU