Recce Pharmaceuticals Ltd (ASX:RCE, OTC:RECEF) has welcomed positive clinical responses to a new gel formulation of its R327® drug candidate, covered by the Therapeutic Goods Administration’s (TGA) Special Access Scheme (SAS).
Anecdotal results from two patient case studies are a positive indicator of the gel formulation’s therapeutic potential, while results from an ex vivo burn wound study indicate the R327 gel had the greatest overall efficacy against a strand of antibiotic-resistant bacteria.
Investors have also welcomed the news with shares as much as 24.59% higher within the first half hour of ASX trading to A$0.76.
Recce CEO James Graham said: “Antibiotic resistance is globally recognised as one of the greatest threats to human health today.
“To see Recce making a difference to patients in such great medical need before us, is another welcomed sign of new hope in the fight against drug-resistant superbugs.
“We look forward to building upon these successes among the present and future clinical trials ahead.”
Case studies
Recce’s R327 gel formulation was administered under SAS Category A — a notification pathway that can be accessed by health practitioners for patients who are seriously ill.
Patients typically have conditions from which death is reasonably likely to occur in a matter of months, or from which premature death is reasonably likely to occur in the absence of early treatment.
The first publicised case study involved a male in his early 70s, who was suffering a puncture wound on his foot from a metal spike.
The patient’s injury was unresponsive to all prior antibiotics, meaning infection was spreading and his medical team needed to prepare for surgical intervention.
However, after 24 hours — and with only one dosing application of the R327 gel — the infection had clinically responded and no pre-treatment wound debridement was needed.
The wound’s redness and swelling also reduced, and at 30 days post-treatment and successfully healed and closed.
In a second case study, a 72-year-old male with type two diabetes was suffering from peripheral vascular disease and neuropathy. Before receiving the R327 gel, he was also unresponsive to all prior antibiotics.
Seven days after the gel formulation was applied, initial redness and swelling had minimised and the wound began to heal and dry up.
By day 10, the wound showed no signs of infection or pus formation and continued to clear up and heal.
Two weeks after the R327 gel treatment, the wound has significantly improved and the formulation was well tolerated, meaning the team could avoid surgical intervention (which commonly involves limb amputation thin diabetic patients).
Other case studies have been not disclosed to protect patient confidentiality, but the R327 gel was also successful in combatting flesh-eating bacteria, bone infection and complex skin structure bacterial infections.
Background research
In other news, an independent clinical organisation conducted an animal study where multiple R327 gel formulations were tested for efficacy against Methicillin-resistant Staphylococcus aureus (MRSA).
This kind of bacteria is listed high on the World Health Organisation’s Priority Pathogen list of antibiotic-resistant bacteria, meaning it’s an area where new antibiotics are urgently needed.
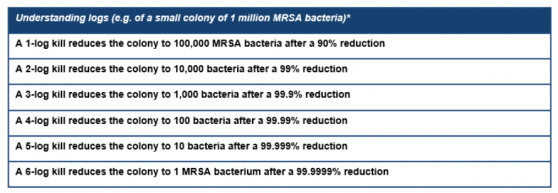
The researchers used an ex-vivo pig skin model, and after 24 hours, the R327 gel had achieved a 4 to 5-log reduction (99.99% - 99.999% reduction) in all formulations and had the greatest overall efficacy against MRSA.
MRSA infections are one of the leading causes of hospital-acquired infections — they are commonly associated with significant morbidity, mortality, length of stay and cost burden.
This kind of antibiotic-resistant bacteria most often causes skin infections and, if left untreated, can become severe and cause sepsis.
Amid the encouraging case study and research results, Recce is advancing clinical trial applications to evaluate the R327 gel’s efficacy in common and complicated skin and soft tissue infections in the lab.
It’s important to note that RECCE® 327 is not yet market-approved for use in humans, with further clinical testing required to fully evaluate the drug’s safety and efficacy.