Recce Pharmaceuticals Ltd (ASX:RCE, OTC:RECEF) is celebrating today after an independent body verified positive Phase 1 clinical data for its lead RECCE® 327 (R327) drug candidate.
The healthcare stock is developing a new class of synthetic anti-infectives and recently conducted a randomised, placebo-controlled, first-in-human trial to evaluate how R327 performed as an intravenous infusion formulation in 80 healthy male subjects.
Since then, independent examiners have looked over data from the 80-person trial, finding all participants achieved the primary study end-points and met international regulatory data standards, indicating R327 is safe and well tolerated.
What does it all mean?
Recce non-executive director and the trial’s medical monitor, Alan Dunton, said: “We are pleased to see that, even when administered at doses much higher than the expected therapeutic window, R327 IV does not lead to safety or toxicity issues in healthy subjects.
“We believe in the potential of R327 to provide a much-needed solution to patients with serious infections and look forward to providing interim proof-of-efficacy data in presepsis patients in the second half of 2023.”
Chairman John Prendergast echoed his comments: “We are highly encouraged by the safety and tolerability R327 demonstrated across eight cohorts at doses up to 6,000 mg, which is above the expected therapeutic range.
“We look forward to building off these results by initiating a Phase II study in patients with early-stage sepsis."
Investors have joined the celebrations with shares as much as much as 17.17% higher in ASX trading to A$0.785.
What are the key findings?
Recce’s Phase 1 R327 trial reported no serious adverse effects or deaths, nor any significant changes in clinically significant hematology or chemistry parameters.
The same goes for other key clinical parameters, such as coagulation, urinalysis and vital signs monitoring. Recce also reported that there were no cardiac events or abnormalities over the course of the trial.
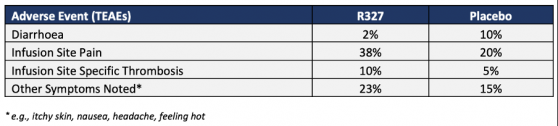
Overall, R327 was found to be well tolerated with a good safety profile across all dose groups from 50 milligrams to 6,000 milligrams when administrated intravenously over one-hour infusion.
In addition, all treatment-emergent adverse events (TEAEs) across the cohorts (including placebo groups) were classified as mild or moderate and were ultimately recovered or resolved.
What are the implications?
The Phase 1 findings indicate there is potential to use R327 as a sepsis or urinary tract infection treatment — an area of considerable unmet medical need. UTI’s are responsible for about 30% of all sepsis infections, defined as ‘Urosepsis’.
Prendergast explained: “There are currently no specific treatment options available for sepsis, with patients generally given broad-spectrum antimicrobials at first and then refined once the antibiograms become available a few hours after treatment initiations, which can be critical for patients with sepsis.
“Our next-generation anti-infectives have the potential to change the treatment landscape by becoming a universal first-line treatment for patients with life-threatening infections from both Gram-positive and Gram-negative bacteria, including their superbug forms.”
Interestingly, R327’s clinical results show a significant dose-dependent concentration of R327 in both the urine and the plasma when the concentration of R327 found in the urine was up to 21 times higher than in the plasma.
The trial also found that R327’s primary route of excretion appears to be through the kidney, into the ureters and down into the bladder.
What’s more, an independent study demonstrated R327 in human urine could reduce the number of viable E. coli bacteria by over 99.99% in a matter of minutes.
This, along with the drug candidate’s excellent safety profile and preclinical in-vivo kidney and UTI bacterial infections studies, highlights its potential for treating complicated and uncomplicated UTIs.
Another positive but unexpected finding was the improvement of healthy human cells in the PK/P.D. analysis, including indications of improved kidney health from R327 as it was excreted from the body.
Why is this important?
In most cases, antibiotics can cause kidney damage and can be quite a common occurrence.
For example, antibiotics use in hospitals account for roughly 10% of acute renal failure episodes, as well as 60% of drug-related kidney damage.
Moreover, antibiotics can form crystals that don't break down and block urine flow. One such example is one of the world’s most widely prescribed commercial antibiotic, which generates more than US$10 billion in revenue and is known to cause urine crystallisation.
Others have substances that can damage certain kidney cells when attempting to filter them out.
However, results from Recce’s Phase I study demonstrate no change in any chemistry parameter to those dosed with R327, with all kidney and liver functions appearing to be normal.
Consequently, this could have significant therapeutic implications in the urinary tract infection and sepsis treatment space.
Where to from here?
Recce’s Phase I intravenous study has paved the way for the next stage of R327's clinical development in sepsis and UTIs.
While clinical data verification is hot off the press, the company is wasting no time as it prepares R327 for further clinical development.
Recce is currently conducting a Phase I/II UTI clinical trial evaluating R327 at faster intravenous infusion rates.
The team recently announced it had dosed its first cohort, including the study’s first female subject.
Read more on Proactive Investors AU