Clinical-stage oncology company Prescient Therapeutics Ltd (ASX:PTX) has fielded more promising results from its PTX-100 T Cell Lymphoma Phase 1b Cohort study, targeting relapsed and refractory T cell lymphoma (TCL).
The company says PTX-100 continues to show encouraging clinical activity in this difficult-to-treat patient population, having achieved several clinical responses.
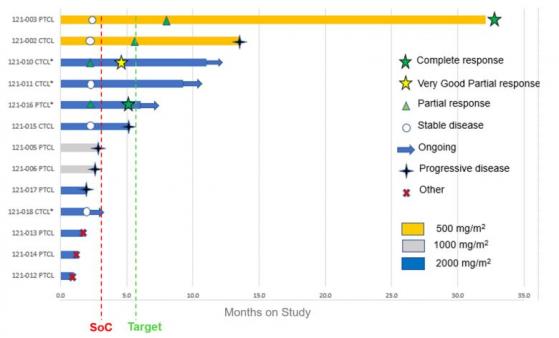
Seven out of 10 patients evaluated had positive responses exceeding those found in the normal standard of care.
Most notably, two patients with relapsed and refractory peripheral TCL have had complete responses (complete eradication of cancer) since the prior update in November 2022, which is not generally expected in this disease.
Swimmer plot of individual TCL patient responses and duration.
Significant unmet therapeutic need
“It is very exciting to see this clinical data for PTX-100 continue to unfold so favourably, especially in these relapsed and refractory T cell lymphomas, which are particularly difficult to treat and where other therapies have failed,” Prescient Therapeutics CEO and managing director Steven Yatomi-Clarke said.
“Unlike other TCL therapies, PTX-100 continues to exhibit an excellent safety profile and the patient responses we are observing are very promising for a Phase 1b study.
“Whilst Phase 1 trials necessarily focus on safety, we have a valuable opportunity to bolster our trial with a small number of additional patients to enable Prescient to have a more meaningful and productive dialogue with the FDA.
“This follows last week’s decision by the FDA to grant PTX-100 Orphan Drug Designation for all TCLs and presents an exciting and unique opportunity for Prescient and for TCL patients awaiting more effective therapies.”
Read: Prescient Therapeutics granted US FDA additional Orphan Drug Designation for PTX-100; shares up
The study is being led by globally renowned haematologist, Professor H Miles Prince at Epworth Hospital in Melbourne, Australia.
Path to registration
Prescient will be applying for a meeting with the FDA later this year.
A favourable outcome would see the registrational Phase 2 study open within 12 months, subject to satisfactory results from the Phase 1b trial.
A possible scenario may involve regulatory interactions taking place and/or a subsequent Phase 2 trial initiated before the current Phase 1b officially concludes, due to the long duration of responses being observed in this Phase 1b study.
If Accelerated Approval is not granted, the Phase 2 trial will proceed as per conventional drug development pathways, with a subsequent study likely required for approval.
Prescient will also be seeking clarification on the dose optimisation and dose schedule considerations for the Phase 2 study pursuant to the FDA’s Project Optimus, which seeks to maximise not only the efficacy of a drug but also its safety and tolerability.
“We are very pleased to see these promising efficacy and safety results in this difficult-to-treat patient population,” Prescient Therapeutics chief medical officer Dr Terrence Chew said.
“With confirmation of these preliminary results, we expect to proceed expeditiously to a registration trial and to be able to provide PTX100 to these patients who desperately need more effective therapies.”
Read more on Proactive Investors AU