Regenerative medicine company Orthocell Ltd (ASX:OCC, OTC:ORHHF) is focused on improving patients’ quality of life by developing and manufacturing collagen medical devices and cell therapies that restore mobility, function and performance.
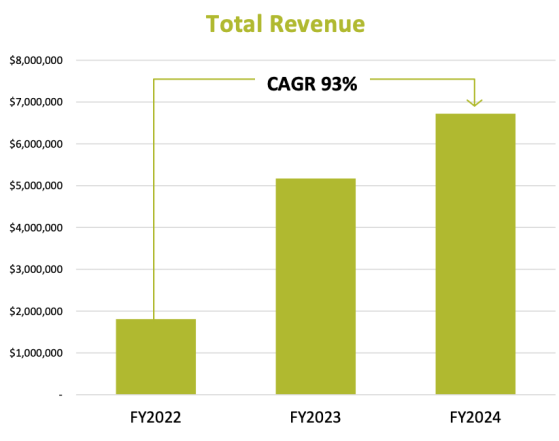
During the 2024 financial year Orthocell reported total revenue of A$6.76 million, up 30.48% from A$5.17 million in FY23.
Along with growing revenues, the company achieved the following key milestones:
- completed the board renewal program;
- strengthened the company’s financial position with A$20.6 million cash at bank;
- solidified a long-term partnership with the University of Western Australia;
- accelerated global market expansion of its medical devices; and
- progressed its pivotal nerve repair study to support US regulatory approval of Remplir™.
Backed by the recent board appointments of John Van Der Wielen and Professor Fiona Wood AM, Orthocell is in a strong position to drive its products into global markets and accelerate revenue growth.
Medical devices - Striate+™ and Remplir™
During the past year, the company accelerated the market expansion of medical devices Striate+™ and Remplir™.
Orthocell accelerated global market expansion plans for both devices during the year with seven regulatory applications, either in progress or planned, in large and attractive new markets.
Striate+™
Striate+, for dental bone generation, is now approved in the US, EU/UK, AUS/NZ and CAN.
Sales to BioHorizons, with whom the company has a global exclusive licence and distribution agreement, continued to build momentum in existing markets during the 2024 financial year.
This traction has resulted from BioHorizon’s comprehensive marketing and medical education programs and the 98.6% success rate observed in the Striate+ post-market clinical study.
BioHorizons has been actively promoting and selling Striate+ to dental surgeons in the US for just over 18 months, launching the device in November 2022.
It has received excellent feedback, with uptake driven by the surgeons’ preference for a high-quality dental membrane that is easier to use and facilitates better patient outcomes.
Remplir™
The second product of Orthocell’s medical device platform, Remplir for peripheral nerve repair, is approved in AUS and NZ.
Sales to Device Technologies (DVT) — the exclusive distributor of Remplir across Australia and New Zealand — continued to build momentum in AUS and NZ during FY24.
This traction was driven by DVT’s comprehensive medical education programs and supported by 85% success rates from the Remplir nerve repair study published in the peer-reviewed Journal of Reconstructive Microsurgery Open.
DVT officially launched Remplir in Australia in November 2022 with a focus on supplying existing orthopaedic and plastic reconstructive KOL accounts.
The ramp-up of product sold in the ~18 months since market launch is gaining traction with 120+ orthopaedic and plastic surgeons now using Remplir in peripheral nerve repair surgeries, from facial nerves to upper and lower limb nerves, across Australia and New Zealand.
Orthocell says feedback is very encouraging, with adoption driven by Remplir’s unique qualities that enable less suturing, creation of the optimal healing microenvironment and facilitation of free gliding within the repair site during the critical healing period.
Orthocell’s global expansion strategy for Remplir continues to build, with the company on track to receive regulatory clearance from the US FDA in 1Q CY25. Regulatory approval for Remplir in Singapore —considered the gateway to other ASEAN markets — is expected in the current half-year.
Cellular therapies - OrthoATI™
Orthocell’s cell therapies aim to treat diseased or damaged tissue by local implantation or injection of healthy cells where tissue repair is needed. Now with US TGA approval, the company intends to engage a strategic partner to accelerate US market access for its leading cellular therapies.
OrthoATI is a world-leading cell therapy developed to treat chronic degenerative tendon injuries (tendinopathy/tendonitis) and can treat tendinopathy in multiple anatomic locations, such as the shoulder, elbow, ankle, knee and hip.
During the year, results from its randomised clinical study comparing OrthoATI to surgery for the treatment of severe, chronic, treatment-resistant lateral epicondylitis confirmed that the study met its primary endpoint, demonstrating that OrthoATI is as effective as surgery in the treatment of lateral epicondylitis.
With two successful randomised controlled studies (in lateral epicondylitis and rotator cuff tendinopathy) now completed, Orthocell is well positioned to engage partners to accelerate US market access.
Read more on Proactive Investors AU