The World Health Organisation (WHO) said last month that “Dengue fever will become a major threat in the southern US, southern Europe and new parts of Africa this decade.”
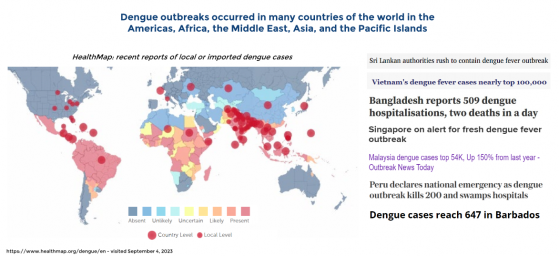
“About half of the world's population is now at risk of dengue with an estimated 100 –400 million infections occurring each year,” according to the WHO.
Unfortunately, Dengue fever is the very definition of an unmet medical need. While millions of humans are infected each year, it is thought that around 30-50% people with the disease do not present with symptoms, enabling the virus to spread within communities.
Warming global climate has accelerated the presence of mosquito-borne viruses resulting in dengue becoming an endemic in more than 100 countries with no current pharmaceutical treatment.
Enter Island Pharmaceuticals Ltd (ASX:ILA).
The drug research and repurposing company is focused on developing preventative or therapeutic drugs for viral infections.
Its lead asset ISLA-101 is being repurposed for the prevention and treatment of dengue fever and other mosquito (or vector) borne diseases.
The company recently passed the final key milestone before beginning a Single Ascending Dose study for ISLA-101 with Human Research Ethics Committee (HREC) approval received.
ILA’s Single Ascending Dose study is a dose escalation study, in which healthy subjects will receive escalating doses of ISLA-101.
If all runs smoothly, ILA’s aim is to have final data by early 2024 and then rapidly transition to the Phase 2 PEACH study soon thereafter.
Read more on Proactive Investors AU