Imugene Ltd (ASX:IMU, OTC:IUGNF) has received the US Food and Drug Administration (FDA) Investigational New Drug (IND) approval to begin a first-in-class clinical study for its oncolytic virotherapy candidate onCARlytics (on-CAR-19, CF33-CD19, HOV4).
In a giant leap forward, the clinical stage immuno-oncology company can now begin patient recruitment and dosing in the clinical study for the onCARlytics platform in patients with solid tumours.
“Imugene receiving this IND clearance for onCARlytics from the FDA is a crucial step forward,” Imugene managing director and chief executive officer Leslie Chong said.
“The start of our onCARlytics study, which is first-in-class, is a significant milestone for clinicians treating patients faced with the challenge of solid tumour cancers, which to date have been untreatable with CD19- targeting biological drugs.
“Accomplishing this goal speaks to the perseverance and dedication of Imugene’s and City of Hope’s research and development teams as we continue to build on our clinical and commercial potential.”
About the study
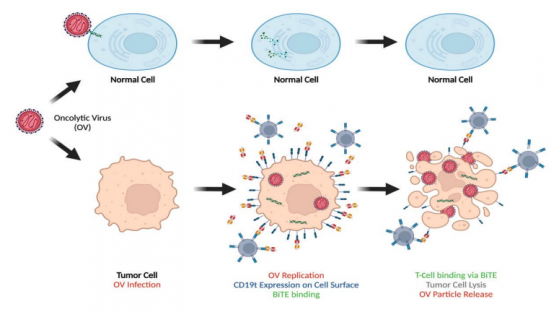
Imugene’s CF33-CD19 oncolytic virus, when combined with the CD19 bispecific antibody blinatumomab (Blincyto®), has the potential to target and eradicate solid tumours that otherwise cannot be treated with Blincyto therapy alone.
In the first-in-human study in adult patients with advanced or metastatic solid tumours, Imugene will evaluate the safety and efficacy of administering CF33-CD19 through intratumoral (IT) injection and intravenous (IV) infusion, either alone or in combination with blinatumomab.
The dose escalating study will be conducted in the United States.
CF33-CD19 selectively infects and drives tumour.
Read more on Proactive Investors AU