Imugene Ltd (ASX:IMU, OTC:IUGNF) has reached the next key milestone in its cancer-killing virus trial.
The clinical-stage immuno-oncology company will escalate the dosage in its Phase 1 metastatic advanced solid tumours (MAST) study, which is evaluating the safety of its VAXINIA therapy candidate.
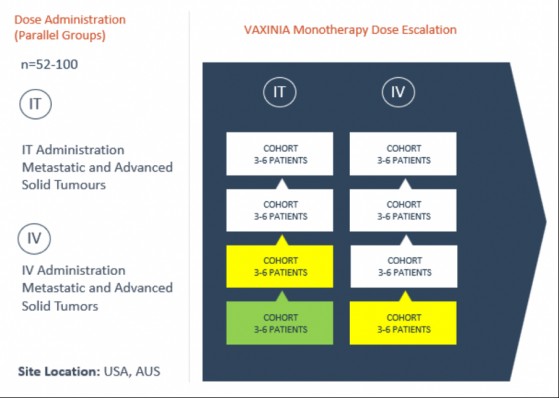
Because the first intratumoral (IT) cohort has now cleared, cohort two is now ready for IT administration. Simultaneously, the first intravenous (IV) cohort is open for dosing.
The dose escalation plan for the Phase 1 VAXINIA trial.
What led to this milestone?
The Cohort Review Committee (CRC) unanimously agreed VAXINIA to be safe, with no dose-limiting toxicities and no serious adverse reactions observed.
The CRC reviewed all safety and tolerability data for the first three patients dosed with the lowest dose of VAXINIA as monotherapy.
With the review meeting under its belt, the CRC advised Imugene to move forward with the second VAXINIA Phase 1 cohort at the mid-dose level.
Speaking to the study’s next step, Imugene managing director and CEO Leslie Chong said: “Our VAXINIA trial has made headway since commencement in May.
“We expect this to continue as site activation and patient recruitment builds momentum and we look forward to updating our stakeholders as this positive progress continues throughout the year.”
Study details
Imugene’s Phase 1 trial is delivering a low dose of VAXINIA to patients with metastatic or advanced solid tumours who have had at least two prior lines of standard of care treatment.
The City of Hope-developed oncolytic virus has been shown to shrink colon, lung, breast, ovarian and pancreatic cancer tumours in pre-clinical laboratory and animal models.
Once patients in the monotherapy group have been treated with the lowest doses of VAXINIA and acceptable safety has been demonstrated, new study participants will receive it in combination with the immunotherapy pembrolizumab.
This is expected to begin once cohort two is cleared per route of administration (IT and IV).
Overall, the study aims to recruit up to 100 patients across roughly 10 trial sites in the United States and Australia.
It’s expected to run for around 24 months and is funded from existing budgets and resources.
Read more on Proactive Investors AU