The Healthcare industry is a many-faceted beast, consisting of everything from biotech heart valve replacements to oncolytic cancer-fighting viruses and psychedelic trauma therapies.
2022 was a bit of flat year for the sector, but early signs point to a stronger showing in 2023.
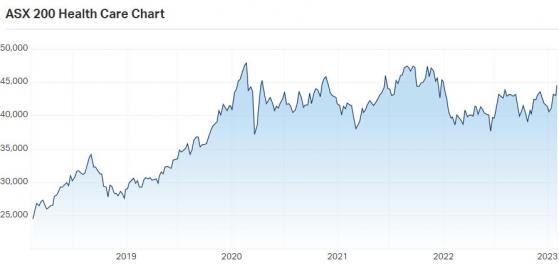
State of the market
Interest in the biotech industry increased dramatically in the face of COVID-19, the first global pandemic the world had seen since the Spanish Flu in 1918, translating into increased awareness and funding that is yet to abate.
ASX200 Healthcare sector chart 2018-2023. Source: Market Index.
While 2022 did see somewhat of a contraction, the market remains highly elevated compared to its pre-2020 levels.
The global market reflects that trend, estimated at US$859.94 billion in 2022 and predicted to grow at a compounding annual growth rate (CAGR) of 8.7% to reach US$ 1,683.52 billion by 2030, according to Precedence Research.
To break the industry down further, the medical device market was valued at US$488.98 billion in 2021, estimated to reach US$718.92 billion by 2029 at a CAGR of 5.5%.
The global oncological or cancer-focused market was valued at US$286.04 billion in 2021 and is expected to reach over US$581.25 billion by 2030 with a CAGR of 8.2%.
The mental health market was valued at $383.31 billion in 2020, and is estimated to reach $537.97 billion by 2030 with a CAGR of 3.5%.
Finally, the psychedelic market, which is still very much in its infancy, was valued at $2 billion in 2020, expected to grow to $10.75 billion by 2027.
That’s a huge CAGR of 27.16%, growth that could potentially outpace the legal cannabis market in the US, according to a peer-reviewed article published by JAMA Network, a medical journal published by the American Medical Association.
In the spotlight: ASX Healthcare stocks
Healthcare is a fast-moving industry that requires a lot of time and capital to produce results, so let’s take a look at who’s making waves in the sector this quarter.
AdAlta
AdAlta Ltd (ASX:1AD) developed a roadmap to Phase 2 clinical trials for its AD-214 i-body platform treatment during the last quarter, secured a second Japanese patent and collaborated with GPCR Therapeutics to explore oncological applications for its platform.
“The December quarter of 2022 saw us collate a year of preclinical and formulation study results and external market data to develop a roadmap to Phase 2 clinical trials and partnering for our lead asset, AD-214. Implementing that roadmap is already well underway,” AdAlta CEO and managing director Dr Tim Oldham said.
1AD’s AD-214 treatment is a first-in-class, next generation antibody therapeutic targeted at the treatment of fibrotic diseases, specifically lung fibrosis, kidney fibrosis, eye fibrosis, and cancer.
Apart from the new potential for cancer applications, the University of Western Australia (UWA) also published results of a study demonstrating an i-body binding to ‘RANKL’ had potential as a new therapy for osteoporosis, a US$8 billion market.
AdAlta has since filed a patent to protect this application, and intends to continue to support UWA’s research.
Finally, the company is in a solid cash position, holding $7.34 million as of December 31 2022 as major shareholder Yuuwa Capital completed a planned end of fund wind-up.
“Yuuwa’s support over many years is greatly appreciated by the company and we thank Yuuwa’s shareholders for their continued confidence in our long-term growth potential,” Dr Oldham commented.
Anteris Technologies
Anteris Technologies Ltd (ASX:AVR, OTC:AMEUF) marked an important milestone this quarter, securing US Federal Drug Administration (FDA) approval for an early feasibility study (EFS) on the company’s DurAVRTM THV System – an aortic valve technology engineered to last the patient’s lifetime.
The company also presented detailed 30-day outcomes for a combined cohort of 13 patients from the DurAVRTM THV First-In-Human (FIH) trial at the PCR London Valves conference.
The technology demonstrated 100% procedural success, as well as “excellent” haemodynamics (blood flow) and restoration of normal laminar (smooth and even) flow dynamics.
Anteris held some $13.8 million in cash at the end of the quarter, shortly after which AVR released one-year follow-up results on the first cohort of five patients, demonstrating what the company describes as “impressive and preserved valve performance with excellent safety".
Artrya
Artrya Ltd (ASX:AYA) achieved a raft of regulatory approvals for its patented artificial intelligence platform designed to provide advanced cardiovascular modelling this quarter, a vitally important step in commercialisation.
The company secured approval from Europe and the UK, which represents AYA’s largest market opportunity to-date.
The assessment scope of Artrya’s European Notified Body (BSI) includes UKCA certification in accordance with the UK Medical Devices Regulations 2002.
The company’s Salix Artificial Intelligence coronary software met or exceeded all regulatory requirements.
With a four-year contract in place to supply the National Health Service Trust Hospitals throughout the UK with the SCA product, AYA is well positioned to take advantage of the new market opportunity.
Looking ahead, Artrya is planning the commercial release of its technology in Australia during this financial year, cashed up with some A$26.8 million to achieve it.
Radiopharm Theranostics
Radiopharm Theranostics Ltd (ASX:RAD) gained Investigational New Drug approval for its RAD301 cancer imaging technology, and presented data on its Pivalate phase 2A imaging trial in brain metastases this quarter, making robust progress in its clinical ambitions.
The company’s HER2 nanobody (RAD201) also demonstrated favourable tumour targeting in breast cancer patients, and Nature – a pre-eminent scientific journal – published encouraging data on DUNP19 receptors role in cancer growth, an indication RAD is targeting.
Radiopharm also secured an important agreement with the Australian Nuclear Science and Technology Organisation (ANSTO) for the supply of key isotope lutetium-177 for Australian trials, and with NorthStar Medical Radioisotopes for the supply of non-carrier added (n.c.a.) Actinium-225.
The lutetium-177 isotope will be used by in combination with Radiopharm’s proprietary nanobody in a Phase 1 therapeutic dose escalation trial in patients with non-small cell lung cancer.
RAD plans to start the trial the second quarter of this calendar year in collaboration with GenesisCare and ANSTO.
Patrys
Patrys Ltd (ASX:PAB) made progress toward Phase 1 clinical trials for its PAT-DX1 treatment in two species, targeted for the second half of this calendar year once the remaining GLP toxicology studies are complete, likely in the second quarter.
New pre-clinical data for the PAT-DX3 treatment also revealed its ability to cross the blood-brain barrier in healthy animals, opening up potential applications for delivery in neurological conditions.
“During the quarter we made significant progress in the development of our deoxymabs for our own programs as well for other applications that may provide commercial partnership opportunities,” Patrys CEO and managing director Dr James Campbell said.
“We remain clearly focused on getting PAT-DX1 into the clinic during 2023 but, in parallel, want to ensure we leverage the range of development opportunities for our deoxymab technology.
“These include developing a GMP production process for our second deoxymab asset, PAT-DX3, which we believe offers a range of additional applications due to its larger size and IgG format.
“In parallel, we have shown the potential for PAT-DX3 to be used for drug-delivery in neurological applications and are currently generating data to support its use for the delivery of gene-editing constructs and the use of deoxymabs as a synthetic lethality treatment for certain cancers.
“This highlights the range of opportunities that deoxymabs offers to develop new therapeutic approaches for treating different diseases.”
Imugene
Imugene Ltd (ASX:IMU, OTC:IUGNF) made several presentations at well-respected healthcare conferences this quarter, as well as material progress on its VAXINIA trial, dosing the first patient in the second intravenous cohort assessing the safety of novel cancer-killing virus CF33-hNIS.
The company made presentation at the following conferences:
- J.P. Morgan Healthcare Conference: selected to present;
- Society for Immunotherapy of Cancer 2022 Annual General Meeting: onCARlytics (CF33-CD19) oncolytic virus combinations presented;
- 2022 San Antonio Breast Cancer Symposium: new and first CHECKvacc data presented;
- ASCO Gastrointestinal Cancers Symposium: HER-Vaxx & CF33 platforms featured; and
- ESMO Asia: HERIZON data presented.
With some $161.9 million in cash or equivalents, the company is well funded to advance its oncolytic virus platform.
Emyria
Emyria Ltd (ASX:EMD)’s highlight for this quarter may well have been its ultra-pure cannabinoid treatment’s acceptance into the US National Institute of Health’s (NIH) Preclinical Screening Platform for Pain (PSPP) program.
Part of the HEAL Initiative and led by the National Institute of Neurological Disorders and Stroke (NINDS), the program seeks to improve prevention and treatment strategies for opioid misuse and addiction, as well as enhancing pain management.
Emyria is developing ultra-pure cannabinoid dose forms suitable for multiple indications, and a proprietary MDMA-analogue library with its partner, University of Western Sydney, supported by its Proprietary Real-World Data platform, which gathers ethically-sourced clinical data from the company’s specialist clinical service.
EMD brought its MDMA-analogue library to more than 140 compounds this quarter, shipping 14 for safety screening and five for advanced preclinical screening.
The company also received ethics approval for a pivotal Phase 3 clinical trial assessing the EMD-RX5 (cannabinoid) treatment’s effect on symptoms of psychological distress, which began dosing in January.
“We closed 2022 with a lot of momentum in our drug development programs with the successful initiation of recruitment for our Phase 3 clinical trial for our leading asset, EMD-RX5, and the expansion of our preclinical R&D pipeline of MDMA analogues,” Emyria managing director Dr Michael Winlo said.
“Emyria also strengthened its network of world-leading research partners via acceptance into a prestigious program managed by the NIH and we boosted our cash position with the closing of a $3 million placement setting us up for a strong 2023 and beyond.”
Incannex Healthcare
Incannex Healthcare Ltd (ASX:IHL, NASDAQ:IXHL) advanced several US Federal Drug Administration (FDA) focused programs for cannabinoid pharmaceutical products and psychedelic medicine therapies this quarter, engaging in a constructive pre-Investigational New Drug Application meeting with the FDA for IHL-216A.
IHL-216A is Incannex’s proprietary combination of cannabidiol (CBD) and isoflurane (ISO) that the company is developing for treatment of concussion and traumatic brain injury (TBI).
Incannex has engaged Curia Global, Inc. to optimise and manufacture GMP-grade IHL-216A in compliance with Current Good Manufacturing Practice (GMP), while also generating data on the quality and stability of IHL-216A to support future regulatory filings and clinical trials.
Apart from IHL-216A, IHL advanced IHL-675A, a multi-use anti-inflammatory drug, to Phase 2 clinical trials, and began a Bioavailability and Bioequivalence study for IHL-42X, targeted at obstructive sleep apnea.
Incannex also expanded its intellectual property position over the IHL-42X patent, expanding the protected uses of the treatment to obstructive sleep apnea and engaged Eurofins to manufacture its novel CBD addiction treatments CannQuit-N and CannQuit-O for nicotine and opioid addictions.
Finally, the company began an independent data review for its Phase 2 trial of psilocybin-assisted psychotherapy for anxiety after completing 29 patient primary endpoint assessments.
MGC Pharma
MGC Pharmaceuticals Ltd (LSE:MXC, OTC:MGCLF, ASX:MXC) continued to advance its CimetrATM and ArtemiCTM platforms during the quarter, demonstrating efficacy and the anti-inflammatory effects of the former in ongoing studies that will inform a US FDA Investigational New Drug submission.
The company also completed a clinical study assessing ArtemiC’s effect on patients suffering from Long COVID, which demonstrated the treatment’s ability to alleviate physical symptoms and mental confusion.
On the corporate side of things, MXC expanded key US and EU commercial partnerships, delivering an ArtemiC order valued at $1 million to AMC Holdings Inc and upgrading the GMP certifications of its Slovenian production and research facility.
Finally, the board implemented a strategic review of its business operations, which resulted in a 35% reduction in director fees and key executive officers agreeing to a 10-20% reduction in their cash renumeration.
Read more on Proactive Investors AU