Emyria Ltd (ASX:EMD) has expanded its proprietary cannabinoid medicine pipeline, with the launch of a high potency, highly bioavailable Ultra-Pure CBD dose form called EMD-RX9, targeting the over-the-counter market.
The launch follows the company’s recent commercialisation success of its low-dose CBD capsule, EMD-RX5 with Aspen Pharmacare (JSE:APN) in Australia.
EMD-RX9 is an advanced formulation capable of holding greater than 150 milligrams of CBD per capsule and will underpin Emyria’s global clinical development plans targeting prescription-based registrations.
Moving forward, the company is advancing a comprehensive US registration strategy for EMD-RX9, comprising pre-IND (investigational new drug) preparations and a head-to-head phase one clinical trial with Epidyolex, planned for the second half of this year.
Superior bioavailability
Emyria CEO Dr Michael Winlo said, "The launch of EMD-RX9 builds on the recent commercialisation success of our low-dose CBD capsule, EMD-RX5 targeting the over-the-counter market.
“The higher dose strength and potency of EMD-RX9 will support Emyria’s US-focused registration programs by helping address the needs of patients requiring high CBD doses.
“We are confident EMD-RX9 will demonstrate superior bioavailability compared to Epidyolex oil based on earlier head-to-head preclinical PK studies performed with EMD-RX7 and, together with our Real-World Data insights, can help Emyria’s growing Ultra-Pure cannabinoid product pipeline address the unmet needs of large patient populations."
Forward plan
Emyria is planning the next steps in EMD-RX9's clinical development including conducting a phase one head-to-head pharmacokinetic (PK) study against Epidyolex, the only registered CBD medication worldwide.
The phase 1 study will help demonstrate EMD-RX9's relative bioavailability advantages and support Emyria’s Pre-IND (Investigational New Drug) meetings in planning.
A Pre-IND is a critical step in the US registration process that can streamline the pathway to further clinical trials and eventual market approval for EMD-RX9.
With these strategic initiatives in place, Emyria is well-positioned to advance EMD-RX9 through the necessary regulatory stages, unlocking new market opportunities and reinforcing the company's commitment to delivering innovative, high-quality cannabinoid-based medicine to patients in need.
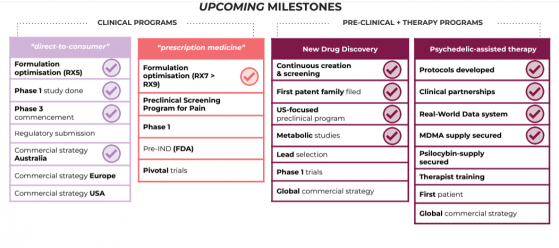
Upcoming milestones
Read more on Proactive Investors AU