Emyria Ltd (ASX:EMD) will collaborate with The Pax Centre (PAX) to develop a scalable care program for the safe provision of MDMA-assisted therapy for patients with treatment-resistant PTSD.
This collaboration with Australia’s leading multidisciplinary, psychiatrist-led clinical service specialising in complex trauma treatment, feeds into EMD’s long-term goal to establish a network of trained therapists and psychiatrists to provide MDMA-assisted therapy.
It is doing so via ethics-approved clinical trials and the Authorised Prescriber pathway, that will lead to appropriate diagnosis of patients from July 1, 2023. The date is important as this is when the Therapeutic Goods Administration (TGA) reschedules MDMA and psilocybin to Schedule 8 (Controlled Drugs).
The caveat as stated by the Royal Australian and New Zealand College of Psychiatrists is that “prescribing psychiatrists must demonstrate adequate training, evidence-based treatment protocols, patient selection and monitoring to be approved to prescribe this therapy. Ongoing psychotherapeutic support is also essential for this treatment model”.
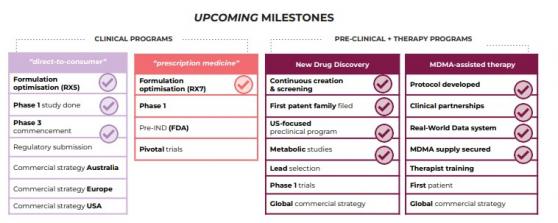
In keeping with this, specialist psychiatrists, trained therapists and facilities will be provided by PAX, while EMD will manage the clinical trial design, Real-World Data generation, data monitoring and MDMA supply made easier by the recent securing of patient-ready MDMA.
Supply secured
On February 13, EMD signed an agreement with Canadian manufacturer PharmAla for the supply of clinical-grade MDMA, facilitated by Mind Medicine Australia ahead of the July 1 milestone.
Emyria is working to build a network of research-oriented therapists and psychiatrists to fulfil its aims. The company believes a comprehensive clinical package (eg program + drug supply) has the potential to be licensed to other specialist groups that are looking to help support the nearly 500,000 Australian patients with treatment-resistant PTSD.
In the US, approximately 10 million people are estimated to suffer from PTSD, a problem that costs the economy around $232.2 billion per year.
In Australia, approximately 1 million people are estimated to suffer from PTSD.
In both regions around 30-50% of PTSD patients have shown resistance to standard treatment.
“As the burden of PTSD grows, the interest and demand for new treatment options increases amongst patients and specialists,” Emyria’s managing director Dr Michael Winlo said.
“Following the TGA’s recent decision to reschedule MDMA to a controlled medicine, we believe the demand amongst psychiatry groups for a safe, cost-effective and evidence-based care program to deliver MDMA-assisted therapy in the community for suitably screened patients.
“Emyria is thrilled to collaborate with The Pax Centre, a leading psychiatric clinical service, to develop this care program.
"Our team looks forward to developing clinical protocols with their experts that may help patients with PTSD while generating Real World Data that can support ongoing program improvement as well as Emyria’s novel drug development program.”
Authorised Prescriber status
Emyria, acting as sponsor, and PAX will start the care program as an ethics-approved clinical trial until PAX psychiatrists are given Authorised Prescriber status and a cost model has been developed.
This would be important given the growth of PTSD throughout the US and Australia alone.
With MDMA-assisted therapy showing promising results for patients with PTSD, the work is ongoing to improve research settings that have been limited due to the need for extensive preparations including therapist training, clinical protocol design, facility preparation, data monitoring and drug supply coordination.
On July 1 this will change as the TGA’s Authorised Prescriber Program in Australia will allow patient access to MDMA-assisted therapy as a Schedule 8 controlled medicine.
Emyria's partnership with PAX aims to directly address these challenges by developing a scalable care program for MDMA-assisted therapy that can be safely and cost-effectively administered to patients with PTSD through community psychiatry groups.
"This collaboration strongly aligns with our mission to provide the best possible care and outcomes for our patients and to continually seek out and adopt new, evidence-based treatments,” PAX centre director, mental health nurse Claire Kullack said.
“As one of the leading services tackling complex trauma in Australia, we have always prided ourselves on being at the forefront of innovative and multidisciplinary approaches to care. We are excited to partner with Emyria to explore the feasibility of this promising new treatment option for our patients with PTSD."
Real World Data
Emyria’s Real World Data will support ongoing care model improvements, crucial cost-effectiveness studies to support reimbursement and its active MDMA-inspired drug development program with its partner, the University of Western Australia.
Through the drug discovery program, Emyria is aiming to create new medicines. It is currently working on:
- faster-acting MDMA-like compound with the potential to shorten treatment session times and increase the number of patients treated and;
- a novel therapy to ameliorate the symptoms of Parkinson’s disease treatment.
For Emyria, the importance of the TGA’s decision shouldn’t be underestimated as its novel MDMA-inspired drug development program will open a pathway to registration and reimbursement for the novel compounds across a range of neuropsychiatric disorders.
Meanwhile, the company is advancing its drug discovery program in Australia and the United States to identify novel, MDMA-like compounds with therapeutic potential and is collaborating with local clinical partners to improve patient access and ongoing research.
Read more on Proactive Investors AU