Arovella Therapeutics Ltd (ASX:ALA) capped off the December quarter in a “solid financial position” with pro-forma cash and cash equivalents of $4.76 million to continue advancing its iNKT cell therapy platform for cancer treatment towards first-in-human clinical trials.
A key highlight in the quarter was the company expanding its pipeline to target solid tumours by signing an exclusive licence with Sparx Group to develop a 'world-first' CAR-iNKT cell therapy targeting a validated target, Claudin (CLDN) 18.2.
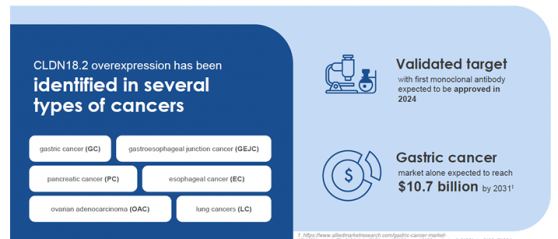
CLDN18.2 is a validated target that is expressed in gastric cancers (GC), gastroesophageal junction cancers (GEJC) and pancreatic cancer (PC).
On the manufacturing front, Arovella completed the GMP manufacturing and release of the ALA-101 lentiviral vector, a key component required for GMP manufacturing of its lead CD-19-targeting iNKT cell product.
Furthermore, the company also continued its manufacturing process development and scale-up activities, with process optimisation remaining on track to support the clinical manufacture of ALA-101 for phase I clinical trials in the first half of 2024.
$ALA Arovella Therapeutics saw share price soar in 2023 as it doubled down on inkt cell therapy for cancer https://t.co/pzVqFAqpRR @WeAreArovella #ALA #ASX #ASXNews— Proactive Australia (@proactive_au) December 29, 2023
Pipeline to target solid tumours
Arovella has also recently secured a global, exclusive licence for the use of a novel monoclonal antibody (mAb) sequence targeting CLDN18.2 in cell therapies.
The mAb, known as SPX-101, has completed all preclinical proof-of-concept, safety and specificity studies and toxicology studies required to commence a phase 1 trial to treat gastric cancers.
Arovella will use the sequence to develop a world-first CAR-iNKT cell therapy targeting CLDN18.2.
Initial proof-of-concept data to demonstrate the potential of this approach is expected to be available in the first half of 2024.
Advancing towards clinical manufacturing
A key requirement for the development of an iNKT cell therapy product is the establishment of the manufacturing process under GMP condition
A critical component for this manufacturing is the GMP-grade lentiviral vector, which carries the genetic material to reprogram iNKT cells to target and eliminate cancer cells.
The vector used to manufacture ALA-101 is a third-generation lentiviral vector manufactured by Lentigen Technology, Inc., a world-leading manufacturer of lentiviral vectors for cell and gene therapies.
In December, Arovella completed the manufacture and release testing of the ALA-101 GMP-grade lentiviral vector.
$ALA Arovella Therapeutics takes key manufacturing step on ALA-101’s path to clinic https://t.co/4h9mfTve4o @WeAreArovella #ALA #ASX #ASXNews— Proactive Australia (@proactive_au) January 2, 2024
New CFO and company secretary
At a corporate level, on December 1, 2023, Arovella appointed Tim Luscombe as chief financial officer (CFO) and company secretary.
Luscombe is a director at Bio101 Financial Advisory, a financial services firm providing outsourced CFO, taxation and company secretarial solutions to the Healthcare sector.
$ALA Arovella Therapeutics appoints new CFO https://t.co/PgKwGq5JH3 @WeAreArovella #ALA #ASX #ASXNews— Proactive Australia (@proactive_au) December 1, 2023
Looking ahead
Over the coming 12 months, Arovella expects to achieve several critical milestones, including:
- presenting initial proof-of-concept data for its new program, CLDN18.2-iNKT cells (H1 CY24);
- manufacturing clinical batches of ALA-101 for phase I clinical trials (H1 CY24);
- completing Investigational New Drug (IND)-enabling non-clinical safety and efficacy studies (H1 CY24);
- securing an Investigational New Drug (IND) application with the FDA and/or regulatory filing with TGA to conduct a phase I clinical trial in non-Hodgkin’s lymphoma (H2 CY24);
- commence a phase I clinical trial in non-Hodgkin’s lymphoma (H2 CY24); and
- in-license additional technologies to enhance the iNKT cell therapy platform.
Read more on Proactive Investors AU