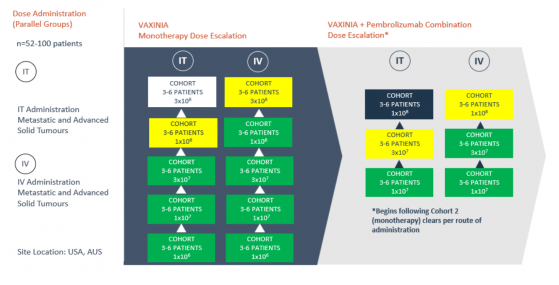
Imugene Ltd (ASX:IMU, OTC:IUGNF) has made progress in its Phase 1 metastatic advanced solid tumours or MAST trial, which has been designed to evaluate the safety and efficacy of the cancer-killing virus CF33-hNIS (VAXINIA).
Intravenous cohort 4 cleared
The trial, which focuses on patients with metastatic or advanced solid tumours, has now successfully cleared cohort 4 of the intravenous (IV) arm of the monotherapy dose escalation study. Cohort 5 is now open for enrolment.
Alongside this, IV cohort 2 of the combination study, where VAXINIA is administered alongside the blockbuster checkpoint inhibitor drug pembrolizumab (KEYTRUDA®), has now been wrapped up and cohort 3 of this combination study is now open.
The Phase 1 MAST trial, conducted at multiple centres, started by administering a low dose of VAXINIA to patients who have previously undergone at least two lines of standard-of-care treatments for their metastatic or advanced solid tumours.
Imugene managing director and CEO Leslie Chong said: “As we near closer to opening and completing the final cohorts that were planned at the beginning of the trial, we have an opportunity to expand the trial by enrolling patients in additional cohorts for the monotherapy dose escalation component.
“This will provide us with a far more robust data set to analyse and speak to at the conclusion of the MAST study, and provide us with a stronger platform as we further the clinical development of CF33 and VAXINIA.”
Tumour-shrinking oncolytic virus
The oncolytic virus, developed by the City of Hope Cancer Center, has demonstrated promising results in preclinical laboratory and animal models, effectively shrinking tumours associated with colon, lung, breast, ovarian and pancreatic cancers.
Overall, the trial aims to enrol up to 100 patients across around ten trial sites in the United States and Australia.
Having started in May 2022, the trial is expected to continue for some 24 months and is funded from existing budgets and resources.
Read more on Proactive Investors AU