Anteris Technologies Ltd (ASX:AVR) (OTCMKTS:AMEUF) (FRA:DDF) is aiming to revolutionise the transcatheter aortic valve replacement (TAVR) space with its up-and-coming heart valve technology.
In an annual general meeting on Thursday, chairman John Seaberg spoke to investors about Anteris’ mission to create durable, effective heart valves.
Following a busy 2020, when the company’s 3D, single-piece aortic heart valve moved from concept to product status, Anteris is eager to spend the coming years achieving the regulatory milestones necessary to get its technology to the market.
Essentially, this means closely working with the Food and Drug Administration to secure a range of approvals, moving the product into more human clinical trials and advancing an early feasibility study.
This year, Anteris’ heart valve will also launch into FDA studies and prepare for regulatory approval work, positioning the technology for commercialisation in the European market.
As it prepares to advance the transcatheter heart valve into the next stage of development, Anteris believes it is well-positioned to capitalise in a market with only two players.
This means a product that operates “significantly better” than current solutions has the potential to reap the lion’s share of rewards in a sector forecast to be worth $10 billion.
Developing DurAVR™ heart valve
In an address at Thursday’s meeting, Seaburg used a visual allegory to describe how the company had evolved its heart valve technology.
“You’ve all heard the story about the sculptor who had made a wonderful sculpture of a bear. The person buying the sculpture asked him ‘how did you take a solid block of granite and turn it into such a beautiful powerful sculpture? It’s easy he said, I just carve away everything that doesn’t look like a bear.
"We’ll, that’s essentially what we have done with our TAVR device. We start with ADAPT® tissue which has great data proving it's less calcific than the current TAVR devices. Less calcific means less brittle over time, which means less breakdown of the leaflets.
“Next, you simplify the design to make it more reliable by taking out 90% of the sutures that the others require.
“Finally, you create a 3D design that has best-in-class hemodynamics. Now if you do that, you can treat not only the really sick aortic stenosis patients, but also be the best treatment for the younger, more active patient. And this is happening during a time when the TAVR market is hungry for new products that will take it to the next level.
“So again, I think we’re standing on the threshold of great value creation. Every large company that touches the TAVR space knows about our DurAVR valve.
“All the signs of value creation are here, including having our valve working very well in humans via surgical placement. It’s my fondest hope and strong belief that we as shareholders will in the not too distant future enjoy the fruits of our investment and our labours.”
Helping patients with aortic stenosis
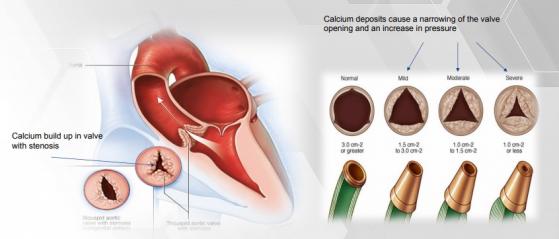
Ultimately, Anteris’ technology will support patients with aortic stenosis — a serious and potentially life-threatening condition where the aortic valve narrows and restricts blood flow.
Arterial stenosis in action.
It’s estimated one in eight elderly Australians have aortic stenosis, while 1.5 million are thought to have the condition in the US — with around 500,000 of those patients suffering from severe aortic stenosis.
Without an aortic valve replacement, as many as 50% of patients with severe aortic stenosis will not survive more than an average of two years.
What’s interesting about Anteris’ offering, however, is its durability and performance: because of its composition and structure, younger patients who get the heart valve can enjoy an expanded exercise capacity.
In an interview with Proactive’s Andrew Scott, Anteris Technologies CEO Wayne Paterson said: “Our valve is performing better under higher cardiac output during exercise than the competitor valves are at rest.”
Confidence builds
One of the key takeaways from Anteris’ latest investor presentation is its confidence in its offering.
While the structural heart company is yet to commercialise the durable heart valve, Anteris believes the move into FDA studies is an indication of its product’s quality.
CEO Wayne Paterson said: “It's important to me to get the point across that the product is coming to market.
“There's no speculation around whether we will develop a product or whether that product will get there.
“It's now moving into its FDA studies and will be into its regulatory approval studies for commercialisation.
“On the back of that not only is it viable but the data is so compelling ... it is significantly better than what is currently in the market.”
Chairman Seaburg echoed Paterson's comments in the AGM address.
“I think we are standing on the threshold of great value creation, and by value I mean not just shareholder financial value, but also societal value as measured by lives saved by our TAVR devices and increased quality of life brought to those who receive our devices.”
A heart valve that lasts longer and works better
Anteris’ technology comprises many different components, but there’s one overarching goal: to create a heart valve that lasts longer and works better than the current solutions.
The structural heart company’s TAVR product utilises three key elements: the ADAPT® tissue, the DurAVR™ 3D, single-piece transcatheter heart valve and the ComASUR™ transfemoral delivery system.
The DurAVR transcatheter heart valve.
ADAPT
Anteris’ core ADAPT technology makes up the tissue component of the heart valve. It’s a special type of material that combats calcification, a phenomenon that narrows a patient’s heart valve and increases pressure.
The ADAPT technology has been proven not to calcify across ten years worth of studies, meaning it has already been established as a working anti calcification treatment.
DurAVR
Anteris used this technology to develop the DurAVR product, with a mission to create a durable heart valve that has very few sutures.
Because it’s made of one piece of material, the DurAVR valve is structurally sound and more cost-effective to produce
The heart valve has already been involved with some human surgeries, and promising results to date have strengthened the product’s regulatory pathway, expediting its development.
Anteris is conducting pre-submission work for an early feasibility study with the FDA and is working to secure breakthrough status for its technology.
In addition, a TAVR human study is scheduled to start later this year, with hopes the new results will compound on previous data.
ComASUR
Finally, Anteris has developed the ComASUR catheter with some of the world’s leading cardiologists.
This is a commissural alignment device that can help surgeons most accurately position the heart valve, which expands like a balloon when it is put in place.
With the three key technologies working in tandem, Anteris believes it is well-positioned to transform the TAVR space.
Read more on Proactive Investors AU