Emyria Ltd’s (ASX:EMD) is set to capitalise on its partnership with Cann Group Ltd (ASX:CAN) (OTCMKTS:CNGGF) (FRA:CVJ) to complete pivotal clinical trials for its EMD-003 CBD medicine targeting mental health and to seek accelerated registration of a unique, low-dose, CBD-only capsule with the Therapeutic Goods Administration (TGA).
Successful registration as a Schedule 3 medicine would result in an over-the-counter (OTC), pharmacist-only CBD medicine.
The company will lead the EMD-003 drug development program using Cann Group’s proprietary Gelpell microsphere technology to register a unique capsule form.
“Partnership accelerates drug development”
Emyria managing director and CEO Dr Michael Winlo said: “This partnership greatly accelerates Emyria’s EMD-003 drug development program by combining Emyria’s unique clinical data and drug development expertise with Cann Group’s best-in-class CBD delivery technology.
“Cann Group’s CBD has already completed robust stability testing as well as Phase 1 clinical trials as required by the TGA.
“This allows us to move straight to pivotal clinical outcomes trials saving significant time and money.”
Advantages of collaboration
The collaboration capitalises on a number of registration milestones already met:
- Real-world clinical data collected with over 400 prescriptions already written for target dose form;
- Pivotal trial protocols developed for target indication;
- Phase 1 trials already completed for 10mg and 100mg doses of the capsule; and
- Gelpell has been shown to have 3.5 times the bioavailability of oils (at 100mg) and has large-scale, GMP manufacturing opportunities.
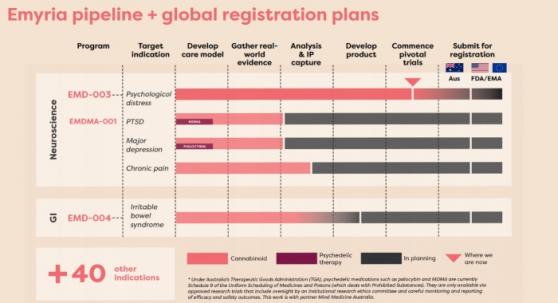
Current drug registration programs also include the EMD-004 CBD +/- THC for irritable bowel syndrome (IBS).
The company recently filed a new IP after extensive analysis of its proprietary clinical data collected with consenting patients, who obtained relief from their IBS condition while being treated at Emyria’s wholly-owned, nationwide clinical subsidiary - Emerald Clinics.
There are currently no approved drug treatments for IBS, which affects around 11% of the population globally and is a significant unmet need often associated with greater levels of anxiety and lower quality of life measures.
The company’s patents cover unique dose strengths and formulations, believed to be most effective in treating a range of IBS-related symptoms including:
- CBD-only formulations, which could support a Schedule 3, OTC registration program in Australia; and
- CBD and THC containing formulations that could support higher schedule applications and international registrations including US Food and Drug Administration (FDA).
Psychedelic-assisted therapy program
The company is also progressing its EMDMA-001 MDMA psychedelic-assisted therapy program with partner Mind Medicine Australia, targeting sufferers of treatment-resistant post-traumatic stress disorder (PTSD).
Pending ethics approval, Emyria will sponsor a major, independently monitored, clinical trial targeting treatment-resistant PTSD with evidence-based MDMA-assisted therapy.
A key priority of this first program is to help evaluate the long-term safety, efficacy and cost benefits of MDMA-assisted psychotherapy.
Such evidence is needed, along with fit-for-purpose clinical infrastructure and trained therapists, to deliver psychedelic-assisted care in a safe, standardised and scalable way.
Pending successful ethics review and the finalisation of all logistics, EMDMA-001 will begin with a Phase II, open-label clinical trial of MDMA-assisted psychotherapy at Emyria’s fit-for-purpose clinic in Melbourne before expanding to other sites.
Emyria’s pipeline and global registration plans.
Milestones for the year ahead
To progress EMD-003 in the next 12 months, the company plans to:
- Commence pivotal registration clinical trials - Contingent on a successful ethics committee review in June 2021;
- Complete trial and commence analysis - Contingent on recruitment success, which is expected four-eight months after commencement;
- Prepare product dossier and clinical evidence package to submit to TGA for registration - expected six-eight months after submission;
- Entry on Australian Register of Therapeutic Goods (ARTG) as a Schedule 3 medication;
- Finalise commercialisation agreements and commence sales as an OTC Schedule 3 medicine; and
- Commence international registration activities.